Z-BaSICTM Computational Chemistry Applications in the Pharmaceutical Industry
Summary
In Z-BaSICTM molecular structure is described by a set of numerical values of carefully defined structural indices. The values of those indices are correlated with activities of pure compounds. This results in a completely digital description of the chemistry of pure compounds. Mixtures are described by an assemblage of pure compounds in a composition/property file, or 'cp' file. Mixture properties are determined additively for those properties that behave ideally and through customized computations for those that do not behave ideally.
The method provides a high level of mathematical rigor and allows for the development of computational tools that are used to extract new knowledge about the activities, properties, and interactions of compounds in biological systems. Applications are seen in pharmaceuticals, biochemical sciences, nutritional sciences, toxicology, and environmental chemistry.
Specifically, Z-BaSICTM is a method for:
What is the Z-BaSICTM Method
Z-BaSICTM is an acronym for the Z-Based Structural Index Correlation Method. Z-BaSICTM is actually a complementary suite of methods for classifying, measuring and correlating properties of chemical substances. It is rigorously founded on principals of thermodynamics and tailored for computational applications. It is NOT a variation of 'group contribution' methods. Group contribution methods often have difficulty with predicting activities of isomeric variations of a set of groups, a problem that has been solved by Z-BaSICTM
.In the Z-BaSICTM method the 'z' number is derived from the classical empirical formula:
CnH2n+z
Where 'z' is a classifying parameter and changes in 'n' are rigorously related to changes in the number of -CH2- groups in the molecule. In actual practice, heteroatoms (N, S, O, P, etc.) are handled by extending the concept to an entire z-vector, but the principal remains the same. Proprietary treatments accurately account for the presence of heteroatoms and isomeric variations of a given 'n' and 'z'.
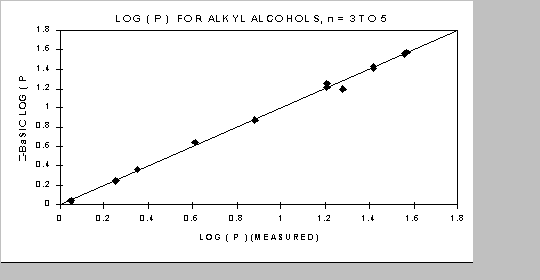
Correlations with properties of interest are greatly simplified by the knowledge that in the infinite 'n' all properties trend to an infinitely long paraffin. This knowledge allows for the selection of correlating equations that behave properly in the regions of unknown behavior. For example, when correlating log P values with Z-BaSICTM structural indices from several points an entire series can be predicted even when structural isomers are present. This is illustrated in the following figure for log P of various alkyl alcohols.
Applications to Mixtures
In the full extension of the Z-BaSICTM method, values for numerical indices can be measured in the presence of other compounds, e.g. mixtures. Using the correlations developed from a few models, the properties/activities of specific compounds can be ascertained without the need to isolate them from the mixture. This feature has obvious benefits for the study of plant extracts, for nutritionals or for discerning the source of activity from impure mixtures such as partially refined synthesis products.
Mixtures are conveniently described by a data file that contains the numerical description of individual components as rows and the properties/activities of those components as columns. This digital array of composition and properties is called a ‘cp’ file. Applications can be written to extract information from the ‘cp’ file or to ‘process’ the file by software programs written to simulate fundamental reaction and mass transport behavior, such as ADME modeling. The technique is especially powerful for those properties/activities that are governed by equilibrium thermodynamics.
Z-BaSICTM and Pharmaceutical Activity
It is well established that biological chemistry is governed in large part by the interaction of molecular species. The biochemical reactions are often controlled by the appearance of a molecular species that does not, itself, participate in a chemical transformation. Discovering and controlling the interaction of molecular species on biochemical reactions is the principal by which most pharmaceutical applications are developed.
Considerations founded in fundamental physical chemistry state that the interaction of a molecular species to a receptor may be described in terms of electronic interactions, geometric structure and conformational effects. When attempting to correlate these interactions with activities (the broad field of QSAR) some progress has been made using the Hammett equation and derivatives of this approach. The Hammett equation, however, falls short of unifying these three effects. In fact, a recent treatise by Hansch and Leo (1995, ACS, p 18-19) points to modern practices that use simplifying assumptions in the relationship between enthalpy (D H) and entropy (D S) that tend to mask the true behavior of intermolecular interactions.
The theoretical foundation of Z-BaSICTM does not make the same simplifying assumptions. Consequently, Z-BaSICTM is able to distinguish between electronic, geometric and conformational effects and this ability provides the foundation for discovery, interpretation, and prediction of biochemically important molecular interactions in greater detail than has heretofore been possible. This capability may become a powerful tool for the emerging science of proteomics.
Business Objective
The economic potential of Z-BaSICTM is enormous. A tool to understand the fundamentals of molecular interactions would lead to accurate predictions of the pharmacological effects, and possible success of clinical trials, of pure compounds. An understanding of the thermodynamics will lead to more rapid understanding of the mechanism of activity. This understanding can occur early in the discovery and research cycle, dramatically shortening the time to trial and improving the selectivity prior to trial. The information may also be useful in predicting the probable outcomes of clinical trials. Additionally, because of the thermodynamic properties contained in the 'cp' file, mathematical modeling of ADME (adsorption, dispersion, metabolism and excretion) dynamics is facilitated.
It is our objective to team with a pharmaceutical, R&D, or service company that would use Z-BaSICTM to reduce their costs, improve value for their clients and customers, and develop the advanced applications of the future. We are open to either exclusive or non-exclusive arrangements relating to defined fields of application.
Please contact Dr. Bunger at
jwba@jwba.com or 801-975-1456 for further discussions.